A Call for Medicaid Programs to Cover SMART for Asthma
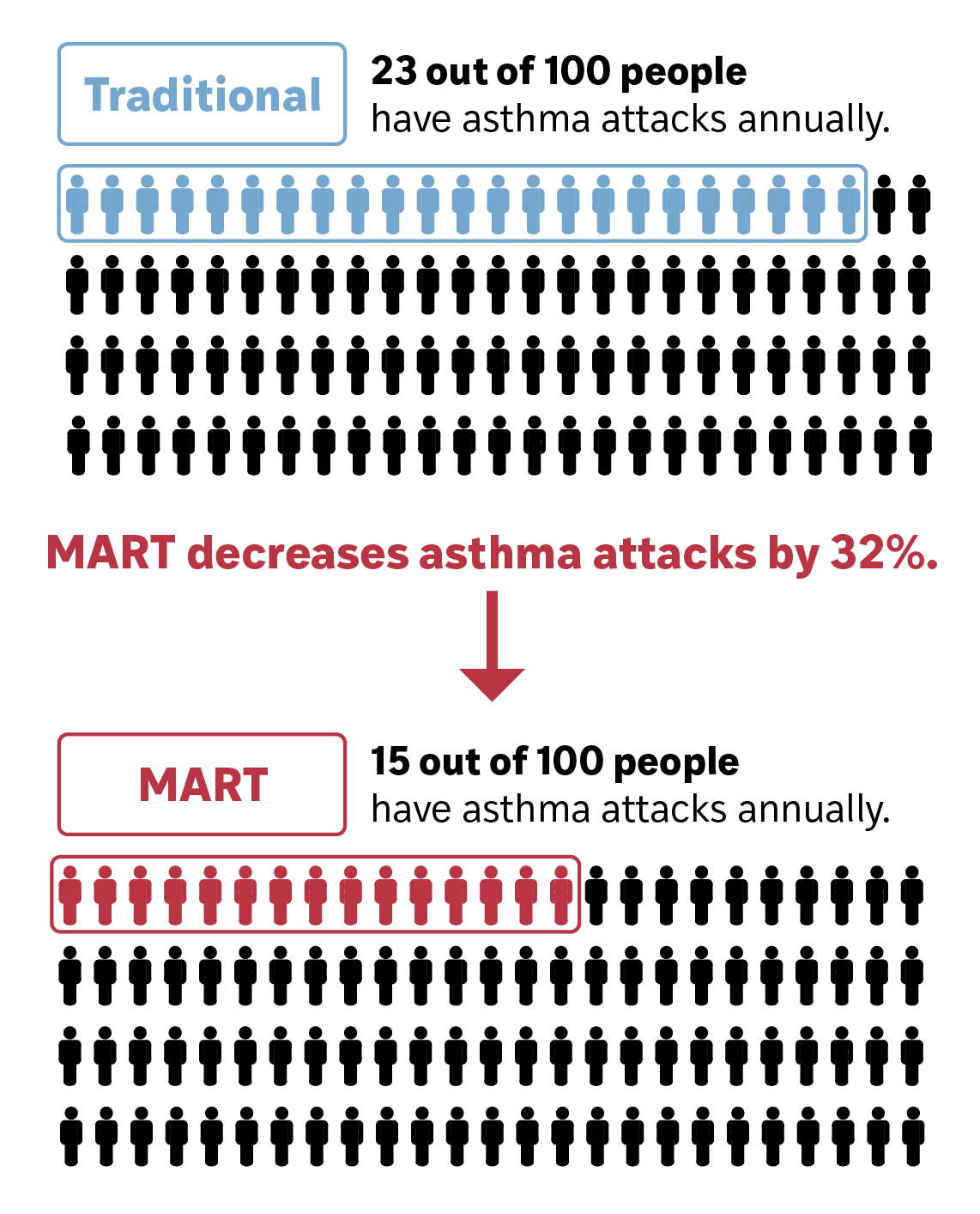
Single Maintenance and Reliever Therapy (SMART) is the guideline-recommended treatment for moderate-to-severe asthma. It uses a single budesonide-formoterol inhaler for both daily (maintenance) and as-needed (reliever) use. Using SMART lowers the risk of severe asthma attacks by about one-third.
Asthma facts
1 in 10 U.S. children have asthma.
26 million Americans
have asthma.
Asthma costs the
U.S. more than $80 billion annually.
About SMART
Guideline recommended by GINA and NAEPP.
Reduces asthma attacks by 30%.
We recommend that all payors:


Include budesonide-formoterol inhalers on their preferred drug formulary.

Cover at least two budesonide-formoterol inhalers per month to support both its maintenance and reliever use.

Ensure budesonide-formoterol inhalers are available with minimal out-of-pocket costs.
Asthma in the United States
Asthma is the second-most common chronic respiratory disease in the United States (U.S.), affecting 1 in 12 people (see state-by-state data here). It is a chronic inflammatory disease that causes wheezing, coughing, and shortness of breath due to airway narrowing and sensitivity.
Each year, about 40% of adults and children with asthma have one or more severe attacks.
Changes in guideline recommendations for SMART prescription


Asthma guidelines have undergone a major shift. In the past, patients with persistent asthma were prescribed two separate inhalers:
A daily maintenance inhaler with an inhaled corticosteroid (ICS)
A reliever inhaler (like albuterol, a short-acting beta-agonist or SABA) for symptoms
However, in real-world use, many patients skip their maintenance ICS inhaler and rely mostly on their SABA reliever — a pattern linked to worse asthma outcomes. Medicaid patients, in particular, have been shown to have lower ICS adherence and higher SABA use.
This is known as SMART (or MART), and it is now guideline recommended by both:
The Global Initiative for Asthma (GINA) ^6
The National Asthma Education and Prevention Program (NAEPP)^8
SMART improves outcomes and is preferred by patients
Strong clinical evidence shows that SMART reduces the risk of severe asthma attacks and is preferred by patients.
Across multiple clinical trials with over 22,000 participants, SMART has proven more effective than traditional asthma treatments. In a recent meta-analysis, SMART reduced the risk of severe asthma exacerbations by about one-third compared to separate maintenance and SABA inhalers (relative risk: 0.68; 95% CI: 0.58–0.80).
Patients also report that SMART is easier to use, helps them feel more in control, and simplifies daily asthma management. It offers similar day-to-day symptom control as traditional regimens — with the added convenience of using only one inhaler for both maintenance and relief.
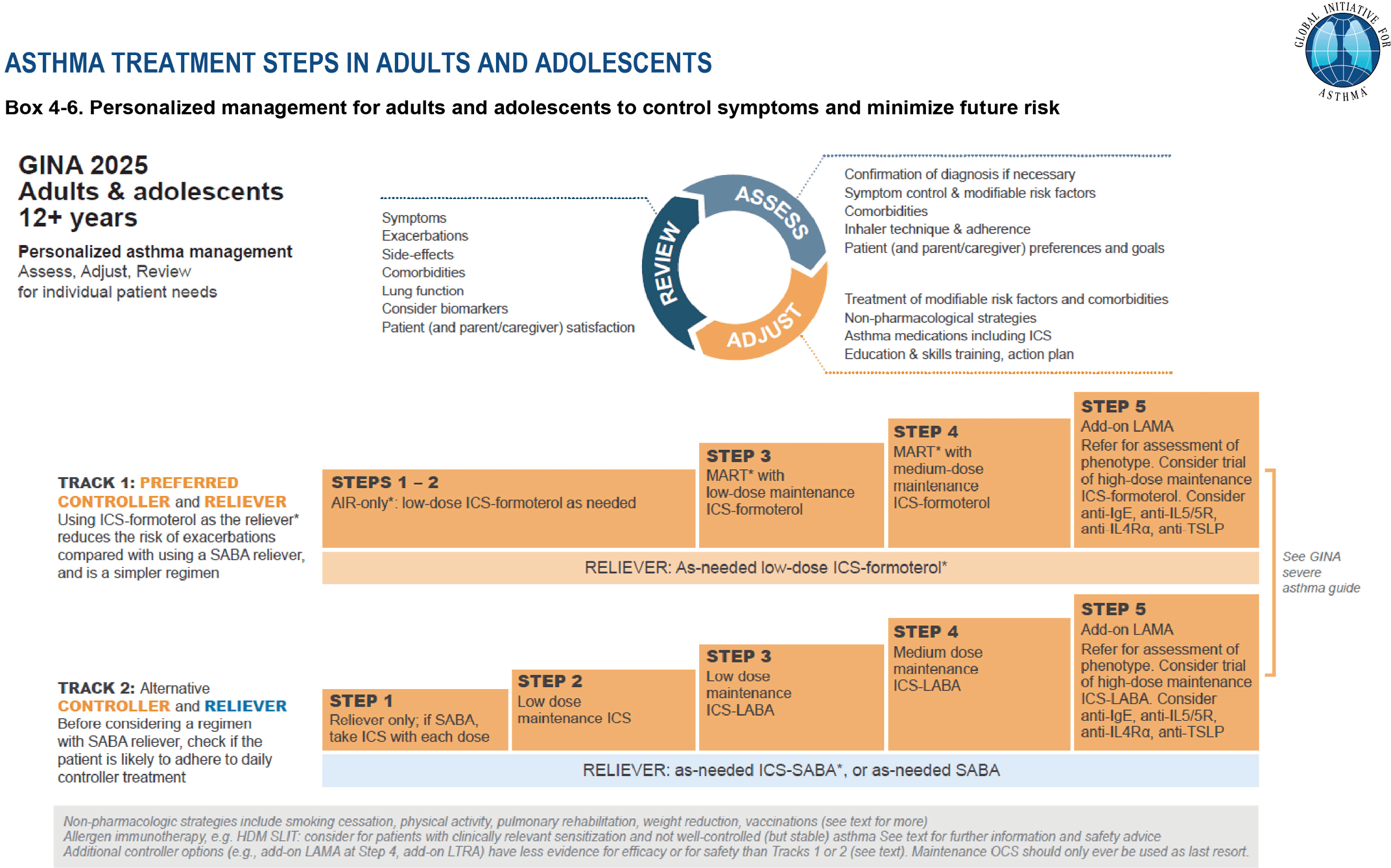
Guideline recommendations for SMART
Both GINA and NAEPP strongly recommend prescribing SMART for patients with moderate-to-severe asthma.
Based on strong evidence of effectiveness and safety, the Global Initiative for Asthma (GINA) in 2019, and the National Asthma Education and Prevention Program (NAEPP) in 2020, began recommending Single Maintenance and Reliever Therapy (SMART) as a preferred treatment.
In the U.S., there are two ICS-formoterol inhalers available that align with SMART:
Budesonide-formoterol
Mometasone-formoterol
Most clinical trials on SMART used budesonide-formoterol, making it the only inhaler explicitly recommended by both GINA and NAEPP for SMART use. Mometasone-formoterol may be a reasonable alternative, though its use in SMART is based on limited or extrapolated data is.
GINA (Global Initiative for Asthma) 2025 recommendations for the management of individuals at least 12 years old with asthma. GINA 2025, reproduced with permission. Available from www.ginasthma.org.
“I like the combined approach [MART therapy] better because I forget to take my [everyday inhaler] so much. And I don’t forget to take my rescue inhaler because my body tells me when I need it.”
–Anne Dixon, MA, BM, BCh
Removing barriers to SMART
Despite the strong evidence and national guidelines support, SMART is still underprescribed.
Although SMART is proven to prevent asthma attacks, most patients still do not receive it. In one study at a large academic center, fewer than 15% of patients with moderate-to-severe asthma were prescribed MART.
A major barrier identified by clinicians is that pharmacy benefit managers (PBMs) often don’t list budesonide-formoterol on preferred drug formularies, making it financially out of reach for many patients.
SMART specifically requires formoterol as the long-acting beta-agonist (LABA) due to its rapid onset, which is essential for fast symptom relief. However, many PBMs don’t distinguish ICS–formoterol from other ICS–LABA inhalers that contain slower-onset LABAs — which should not be used for SMART.
For patients on higher-dose SMART (GINA Step 4–5), the recommendation is:
2 inhalations twice daily, plus
1 inhalation as needed for symptoms
At this dosage, patients will likely run out of a 120-actuation inhaler before the end of the month. Therefore, to support proper SMART use, PBMs should cover up to two ICS–formoterol inhalers per month.
–GINA representative
Is covering SMART worth it? Cost savings for U.S. healthcare payors
SMART is more cost-effective than traditional inhaler therapy because it helps prevent expensive asthma-related complications.
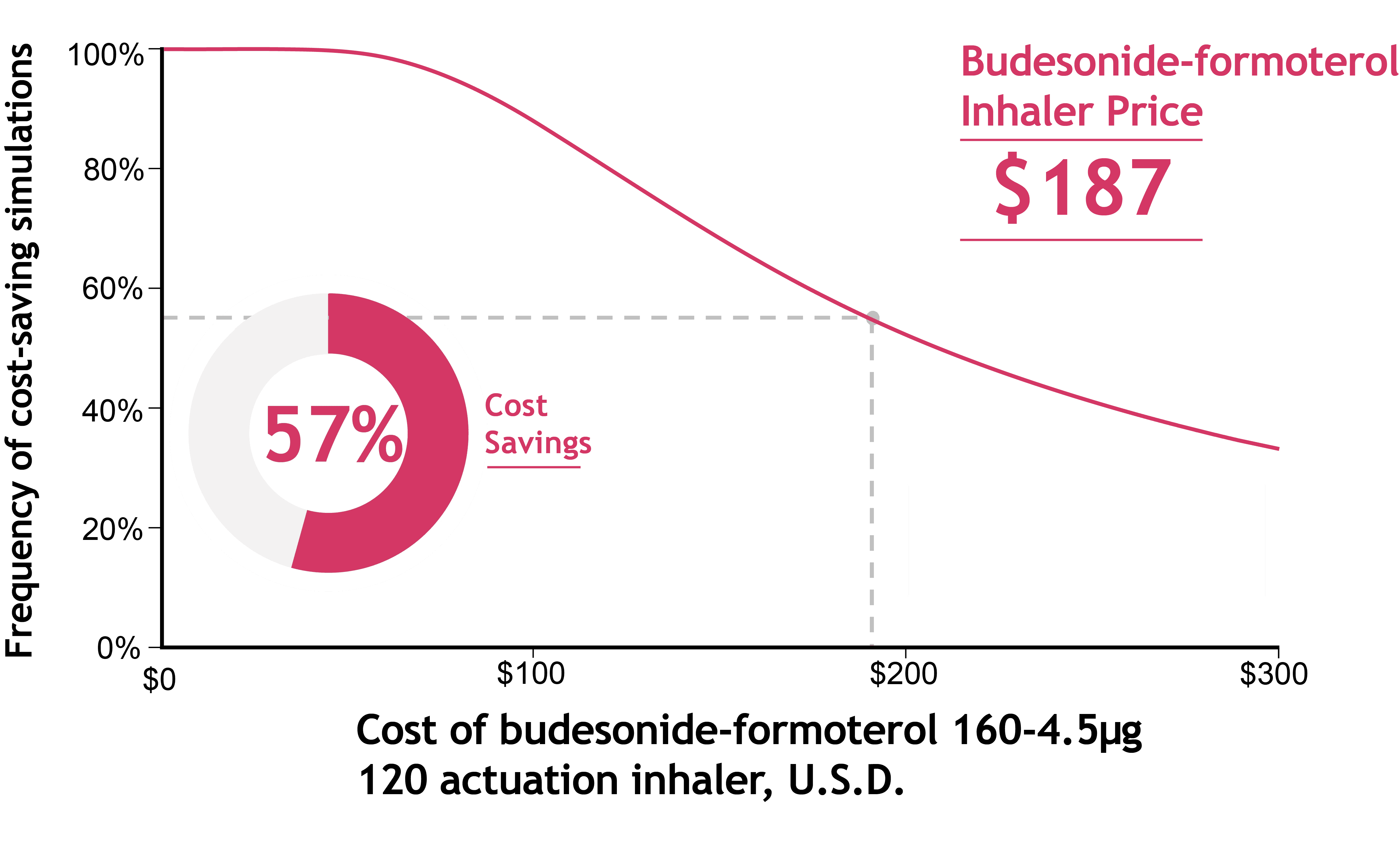
A recent analysis found that if patients were prescribed SMART instead of traditional inhalers, U.S. healthcare payors would save money overall.
Annual savings to payors ranged from $17 to $138 per patient, even after accounting for slightly higher medication costs with SMART.
While SMART may require more frequent inhaler refills, these costs are offset by fewer emergency visits and hospitalizations due to better asthma control.
These analyses calculated one-year direct medical cost savings to payors, but did not include indirect costs like:
Missed work or school
Lost productivity
Early mortality
When these broader factors are considered, SMART is likely even more cost-saving,^31 as it helps patients experience fewer severe asthma attacks and maintain daily functioning.
At an inhaler cost of $187, SMART saved money in 57% of simulations by reducing asthma-related complications.
Use the calculator below to estimate how often SMART is cost-saving at different inhaler prices.
Coverage of SMART within Medicaid by state in 2024
What happens if SMART is covered in your state?

Reduced asthma-related illness and healthcare use:
Multiple clinical trials show SMART reduces the risk of experiencing asthma exacerbations requiring systemic corticosteroids, ER visits, and hospitalizations.

Alignment with national asthma guidelines:
Both GINA and the NAEPP strongly recommend SMART as the preferred therapy for patients with moderate-to-severe asthma.

Simplified inhaler use for patients:
Patients prefer SMART because it is easier to manage a single inhaler for daily control and symptom relief.
Budesonide-formoterol works with a spacer device.
Potential cost savings for Medicaid and health programs:
Recent analyses show SMART generally lowers costs for healthcare payors by preventing expensive emergency or inpatient asthma care.
What we recommend in your state to improve asthma care


Prioritize coverage of budesonide-formoterol
SMART requires formoterol as the LABA due to its rapid onset for quick symptom relief.
We recommend that all states place budesonide-formoterol on their preferred drug list.

Cover at least 2 budesonide-formoterol inhalers each month:
SMART uses the same inhaler for daily maintenance and as-needed relief, so patients often need more than one per monthly.
Therefore, we recommend all payors cover at least two budesonide-formoterol inhalers per month to support proper SMART use.

Reduce out-of-pocket costs for patients:
Studies show that high inhaler costs limit access33, worsen asthma outcomes33, and drive healthcare disparities34.
We recommend minimizing patient co-sharing for SMART whenever possible.
Contact us at:
Dr. James Krings, kringsj@wustl.edu
Krutika Chauhan, c.krutika@wustl.edu
"I like the combined approach [MART therapy] better because I forget to take my [everyday inhaler] so much. And I don't forget to take my rescue inhaler because my body tells me when I need it."
-47-year-old St. Louis resident with asthma who tried MART
This policy brief was made possible through a grant from the American Lung Association (ALA).